Molecular Equation for Copper Sulfate Pentahydrate With Heat
Divide the mass of the water lost by the mass of hydrate and multiply by 100. First of all its CuSO45H2O notice the capital O in the first part of the formula.
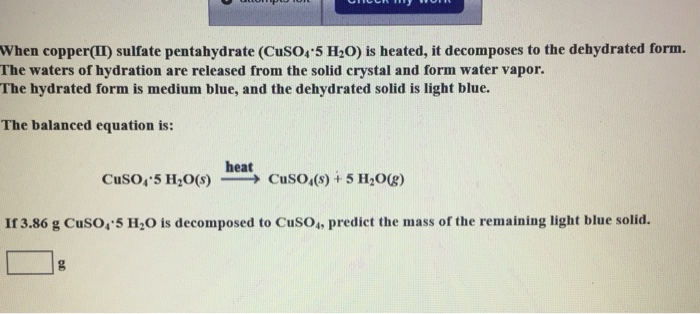
Solved When Copper I Sulfate Pentahydrate Cuso4 5 H2o Is Chegg Com
When copper II sulfate pentahydrate CuSO 4 5H 2 O is heated it decomposes to the dehydrated form.
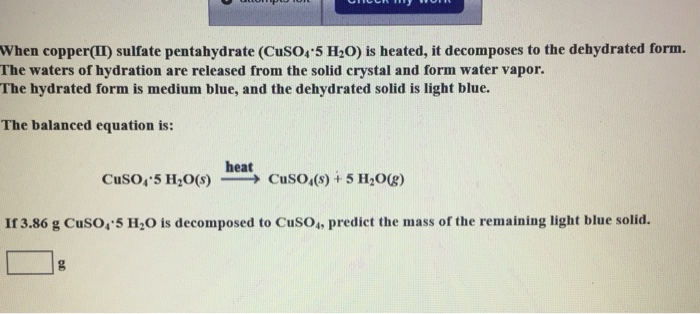
. The balanced equation is. The chemical formula for Copper Sulfate Pentahydrate is CuSO 4 5H 2 O. As soon as the water evaporates in the heating process an sodium chloride anhydrous salt is formed to restore the moisture balance.
It consists of CuSO45H2O in its chemical formula ie. The waters of hydration are released from the solid crystal and form water vapor. Identification of the Substance PRODUCT NAME.
03610 g 1000 g100 3610. Copper II sulfate also known as copper sulphate are the inorganic compounds with the chemical formula CuSO4H2Ox where x can range from 0 to 5. Cu 6355 gmolS 3207 gmolO x 4 1600 gmol x 4 6400 gmolH2O x 5 1802 gmol x 5 9010 gmolNow add.
Copper sulfate pentahydrate CuSO45H2O - PubChem. Cupric copper II sulphate pentahydrate 115. This is because the water molecules are lost on heating and copper sulphate pentahydrate 5 molecules of water H20 is converted to anhydrous copper sulphate no water.
Plano TX 75025 1408 USA TEL. The pentahydrate x 5 is the most common. CuSO 4 5 H 2 Os HEAT ---- CuSO 4 s 5 H 2 O g 1000 g 06390 g.
EcoFusion Inc PO Box 251408. Zn CuSO4 Cu Zn2 SO43. In this regard what is the word equation for copper sulphate.
1 972 403 7449 FAX. So for the classical textbook example of thermal decomposition of copperII sulfate pentahydrate the following steps of dehydration 12 and calcination 34. Click to see full answer.
CuSO45 H2Os ------- CuSO4s 5 H2Og If 242 g CuSO45 H2O is decomposed to CuSO4 predict the mass of the remaining light blue solid in grams. Use EXTREME CAUTION when you are near an open flame Materials. That hydrogen bonding is broken while we heat the compound.
The highly toxic non-combustible odorless blue crystalline powder has a nauseating metallic taste and turns white when dehydrated. Copper Sulfate Pentahydrate DISTRIBUTOR. 400 mL beaker if the hot plate is used or porcelain evaporating dish if Bunsen burner is used 3.
The compound is called copperII sulfate pentahydrate and if we wish to find the percent by mass of water we need to find the molar mass of the hydrate first. Molecular equation for the reaction of copper sulfate pentahydrate with heat states of reactants and products must be shown Molecular equation for the reaction of copper sulfate pentahydrate with states of reactants and products must be shown. 1000 g - 06390 g 03610 g.
Balance CuSO45H2O H2S CuS H2SO4 H2O chemical equation or reaction using this calculator. The structure of the solid pentahydrate reveals a polymeric structure wherein copper is again octahedral but bound to four water ligands. Sulfuric acid copper2 salt.
COPPER SULFATE PENTAHYDRATE 1. CHEMTREC 800 424 9300 2. Chemical PAC-1 PAC-2 PAC-3.
Crop Care Blue Vitriol. Complete and balance the equation. The hydrated form is medium blue and the dehydrated solid is light blue.
Bordeaux copperIIsulfate pentahydrate phyton-27 copperIIsulfate pentahydrate roman vitriol copperIIsulfate pentahydrate sulfacop sulfuric acid copper2 salt 11 pentahydrate sulfuric acid copperIIsalt pentahydrate trianglecopperIIsulfate. There is a balanced equation to use for decomposition of copper II sulfate pentahydrate. H2SO4 Zn CuSO4 H2 Cu SO42- Zn2.
When the crystals copper sulphate is heated for some time the blue colour of it disappears and it becomes white-gray in colour. Ikon copper sulphate pentahydrate. How much heat is required to dehydrate a hydrate.
Hazards Identification Emergency Overview Appearance. When the reaction is complete allow the crucible to cool and then add a drop of water to the product. CopperII sulfate pentahydrate 7758-99-8 12 mgm3.
5H2O----CuSO4 5H2O because the compound containing Cu S O and H is hydrated copper sulfate and has 5 water molecules attached to it. So anhydrous copper sulfate is white in color. The hydrated form is medium blue and the dehydrated solid is light blue.
In this formula two compounds are linked together by a mark. Copper sulfate pentahydrate see results Put a small amount of copper sulfate pentahydrate in a crucible and heat gently with a Bunsen burner. 3 to 5 g of hydrated copperII sulfate CuSO4.
Heat the temperature at only a low to medium temperature 2. Does each step have a chemical equation. 1 214 291 5348 EMERGENCY.
Its CAS is 7758-98-8. So as bond breaking is involved in the reaction we can say that it is a chemical change. It is the following.
Its molecular formula can be given as CuSO45H2O. The difference between the hydrate mass and anhydrate mass is the mass of water lost. Glass stirring rod 4.
Determining the Chemical Formula for Hydrate Safety Precautions.

A What Happens When Copper Sulphate Crystals Are Heated Strongly Explain With The Help Of An Youtube

How To Write The Formula For Copper Ii Sulfate Pentahydrate Youtube

Comments
Post a Comment